Empirical Formula of Butane
Join Login Class 10 General Knowledge Basic Science Basic Chemistry Calculate the empirical formula of butan. The empirical formula for butane is C 4 H 10 When steam reformed C 4 H 10 4 H 2 from CHEM 344 at University of Illinois Chicago.

Butane Molecular Geometry Hybridization Molecular Weight Molecular Formula Cas Number Bond Pairs Lone Pairs Lewis Structure
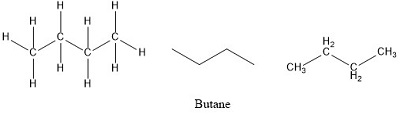
Butane Formula is represented as textC_4textH_10 Butane is typically used as fuel for cigarette lighters and portable stoves a propellant in aerosols heating fuel and a refrigerant.
. The empirical mass of the compound is obtained by adding the molar mass of individual elements. Calculate the empirical formula of butane. Empirical formulas are the simplest form of notation.
Solve Study Textbooks Guides. It is an alkane and consists of four aliphatic carbon atoms in a chain. Thus multiplying 2 to the empirical formula 2 C 2 H 5 C 4 H 10.
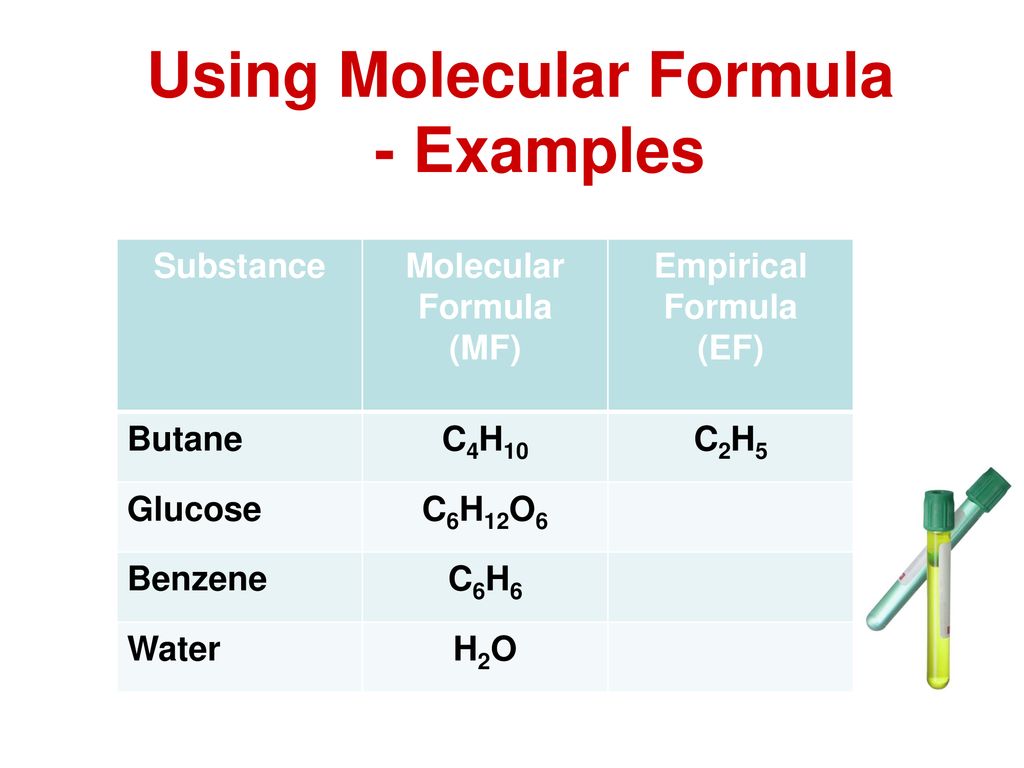
The molecular formula and empirical formula of some substances are the same. For butane and isobutane the empirical formula for both molecules is C2H5 and they share the same molecular formula C4H10. Part A ibuprofen a headache remedy.
The empirical formula for C4 H10 is C2 H5. Steps to determine the empirical formula of a compound STEP 1. What is the empirical formula for butane eqC_4H_10 eq.
In an experiment the molar mass of butane was determined to be 58 gmol. Chemistry questions and answers. C2H3 is the empirical formula for butane C4H6.
The carbon-to-hydrogen ratio equals 23. This gives the empirical formula of butane - C 2 H 5. For every mole of carbon there are two moles of hydrogen.
This is the actual number of atoms of each element in a molecule of butane. The molar mass of the compound is known to us M 5812 g mol 1. The chemical formula of butane is C4H10.
The chemical formula represents the composition of the substance using the. Hence the correct answer is C2H3. In this article lets discuss everything about butane and its chemical formula in detail.
Scroll down to learn more. Calculate the empirical formula of iron oxide. For every mole of carbon there are two moles of hydrogen.
In a chemical reaction 423g of iron reacts completely with 180g of oxygen gas producing iron oxide. Determine the empirical and molecular formulas of each of the following substances. For example butane has an empirical formula of C2H5 lowest whole-number ratio and a molecular formula of C4H10 where the molecular formula corresponds to the molar mass of 5812 gmol.
The carbon-to-hydrogen ratio equals 23. C2H3 is the empirical formula for butane C4H6. Hence the correct answer is C2H3.
C 4 H 8. Butane is a colourless gas that can be easily liquified. This gives the empirical formula of butane - C 2 H 5.
Find the mass STEP 2. Find the simplest ratio. The molecular formula of butane is C 4 H 10.
Butane is a colourless gas and has fair petroleum-like odour. Click hereto get an answer to your question Calculate the empirical formula of butane. This formula does not show the simplest whole number ratio because each number can be divided by 2.
Find the mole STEP 3. They provide the lowest whole-number ratio betweenRead More. What is the molecular formula of butane.
What is the empirical formula of butane. For example both types of formula for carbon dioxide are CO 2. We can simplify the molecular formula C4 H10 which is the formula for butane by dividing the formula What best describes an empirical formula.
However one structural representation for butane is CH3CH2CH2CH3 while isobutane can be described using the structural formula CH33CH. Let the ratio of the molar mass to empirical mass be r. Each C2H5 weighs 2125129.
The empirical formula of butane the fuel used in disposable lighters is C2H5. It is highly flammable and can quickly vapourize at room temperatures.
Empirical Formula Of A Compound Ppt Download

Empirical Formula Flashcards Quizlet

Butane Formula Structure What Is Butane Used For Video Lesson Transcript Study Com
0 Response to "Empirical Formula of Butane"
Post a Comment